Case Studies
PROCESS CONTROL
Restoring Process Control: A Three-Phase Strategy to Mitigate Assay Variability in Solid Dosage Forms
- CHALLENGE
- SOLUTION
- OUTCOME
A large contract manufacturing organization was experiencing out-of-expectation (OOE) results for assay of a solid dosage form. The values were out of control limits for some batches with the risk of out-of-specification results. The product had no history of OOE results. The manufacturing process and analytical procedure had valid states when the quality events were recorded. Previous production batches of the same product had no issues.
Makini Pharma proposed a three-phase plan to mitigate process variability and restore and maintain process control.
Statistical Analysis
- Control charts (x̅, IM-R) were used to trend the assay data of 107 batches and the outcome confirmed the process variability and not isolated incidents of OOE.
- Performed root-cause investigation and it was found that the OOE results were unique to batches of a few campaigns production.
- Data analysis of batches within the campaign revealed that the particle size distribution of a functional excipient used in batches with OOE results was out of trend, although, within specification limits when compared to lots of the material used in batches with trending assay results.
- Makini Pharma worked with the manufacturer and produced 20 batches using a different lot of the functional excipient with trending results for particle size distribution.
- We created OOE limits for particle size distribution using overall standard deviation value and 3σ for monitoring during material lot release. This acted as an indicator signal for OOS that can be captured during material analysis until new specification limits for particle size distribution are set.
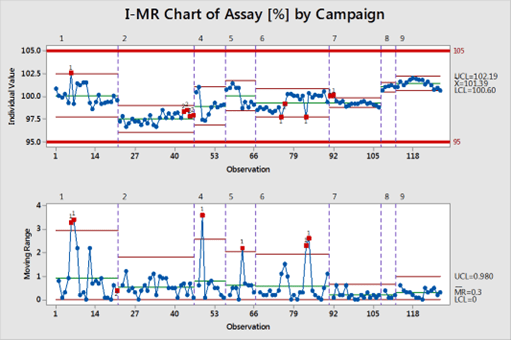
- The state of statistical control was restored for the process as demonstrated by the assay data for the last 20 batches.
- New specifications for particle size distribution set for the functional excipient mitigated the occurrence of the OOE results for assay.
Get in Touch
We're here to answer your questions
Whether you have a question, need further information, or want to explore how our expertise can support your goals, our dedicated team at Makini Pharma is ready to help. Reach out today
